
Understanding the quality of the water in our brooks, streams and rivers has been a central concern of the NRN since its inception. Thanks to a generous grant from Councilor Charles Matthew of the OCC, we were able to acquire professional laboratory equipment to assay quantitatively the levels of chemical pollutants in our water courses and Councilor Dan Levy has kept the lab running by funding the reagents.
Of particular interest to us were the eutrophic pollutants like phosphate and nitrate, but our photometer is very versatile so we can also measure other pollutants, like ammonia and nitrite. We also measure the water’s pH (‘potential of Hydrogen’ – a universal measure of acidity/alkalinity) as well as the dissolved oxygen level, which is crucial for aquatic life.

Dr. Lucy Dickinson leads the water testing program from the front and as a result the NRN now has accumulated 4 years-worth of records from monthly samples taken from 10 sites around Eynsham, with additional samples from South Leigh. The good news? Dissolved oxygen levels and pH are mostly in the typical ranges for open water. The bad news? Levels of the two main eutrophic pollutants - phosphate and nitrate - are way too high. The concentrations vary from site to site and month to month, but the phosphate concentrations are in typically in excess of 1mg/l for most of the sites, where concentrations above 0.2mg/l are classified as ‘poor’. Nitrate-nitrogen concentrations are similarly high, in the range 1 – 5mg/l, where concentrations above 2.0mg/l are classified as ‘poor’.
 Acquiring the data: example of four year’s records for Nitrate concentrations at 10 different sites around Eynsham. Ordinate: Concentration in mg/l. Abscissa: time in months.
Acquiring the data: example of four year’s records for Nitrate concentrations at 10 different sites around Eynsham. Ordinate: Concentration in mg/l. Abscissa: time in months..
Ideally, phosphate levels for good freshwater should less than 0.05mg/l, while nitrate-nitrogen concentrations have to be less than 1 mg/l to be considered excellent. Note that the acceptable levels of nitrate and phosphate for drinking water are typically much higher than the levels necessary for a good aquatic habitat for freshwater plants and animals. To exacerbate water pollution further, we add about 1mg/l of phosphate to drinking water to prevent lead poisoning from old lead pipes (China and Morocco are the sources of the non-renewable phosphate rock used). Since about 25% of our drinking water leaks from the pipes (amounting to nearly 40 liters per person per day), this adds to the phosphate already entering in our water courses from agriculture and waste water.
 Chilbrook ’beasties’ discovered in a ‘Bugs in Brooks’ Workshop.
Chilbrook ’beasties’ discovered in a ‘Bugs in Brooks’ Workshop.
We can see the effects of our degraded water quality by the species of animals that now survive in our brooks and streams. Dr Maarten van Hardenbroek is an expert on aquatic invertebrates and leads the NRN’s ‘Bugs in Brooks’ Workshops where we capture (temporarily) and identify the invertebrates that live in the Fishponds, Chilbrook, Limbrook and Wharf stream. True ‘bugs’ are hard to find, but there are many other fascinating creatures we dredged from the shallows, including leeches, snails, larvae of caddisflies and damselflies, and shrimps.
 Dr. Maarten van Hardenbroek leads a 'Bugs in Brooks' Workshop.
Dr. Maarten van Hardenbroek leads a 'Bugs in Brooks' Workshop.
We have a fun time observing their behavior and sorting and identifying them. Maarten introduced us to the Whalley, Hawkes, Paisley, Trigg (WHPT) metric for assessing river invertebrate communities. It is a metric that captures the quality of the water for aquatic life and was also used by the Environment Agency when they sampled the same watercourses in distant, well-funded times. Like the EA, we also counted the number of species across a number of samples. Putting the EA data together with ours creates a longitudinal record that shows that the habitat for water-life has been deteriorating for years, as the trends in data plotted in the figure below show.
 Historic data from EA and new data from NRN using the WHPT metric (black symbols) and species numbers (brown symbols) for freshwater samples taken in the Wharf stream, Limbrook and Chilbrook.
Historic data from EA and new data from NRN using the WHPT metric (black symbols) and species numbers (brown symbols) for freshwater samples taken in the Wharf stream, Limbrook and Chilbrook.
So why is the quality of our freshwater deteriorating? The headlines in our media point the finger at the legal/illegal release of raw sewage into the rivers by the water companies, but is raw sewage the sole culprit? The simple answer is no – there are multiple sources of pollution. The Institute of Fisheries Management estimates that 40% of the pollution in England is from pesticides, herbicides, and fertilizer from agriculture, 35% is from untreated sewage and 18% is ‘run-off’ from roads and towns, all of which originates from human activities. What is rarely mentioned, however, is that even if there were no release of untreated sewage, the effluent released into rivers by our waste water treatment plants still contains significant levels of pollutants. To understand why this is so, we need to understand what our waste water plants actually do.
Raw sewage contains high concentrations of nutrients, including organic compounds and large concentrations of phosphorus and nitrogen, all of which are ‘eutrophic’ and promote the growth of algae and microbes. Microbes take oxygen from the water for their metabolism and so reduce the levels of dissolved oxygen, which is harmful to aquatic life. This is why we measure the dissolved oxygen concentrations in our water courses as part of our NRN water testing program.
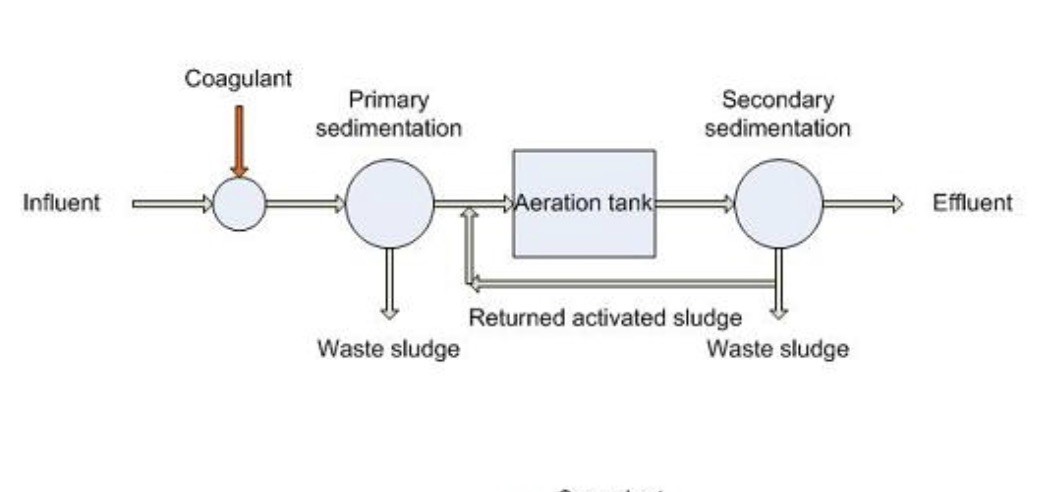 Schematic of activated sludge process for biological treatment of waste water, with an added stage of chemical removal of phosphate This is how Cassington Sewage Works is organized (aerial photo below).
Schematic of activated sludge process for biological treatment of waste water, with an added stage of chemical removal of phosphate This is how Cassington Sewage Works is organized (aerial photo below).
In the UK, most waste water is treated in ‘activated sludge’ reactors (for more details see, A Visit to Cassington Sewage Treatment Works). This process of biological digestion of sewage was pioneered in England and first deployed on an industrial scale in Salford in 1915 and the basic technology has hardly changed since for most UK plants. Activated sludge does two important things: firstly, by aerating the sewage, microbes multiply and ‘eat’ the nutrients, so reducing the biological oxygen demand of the waste water before the settled effluent enters the rivers. Secondly, the microbes convert ammonium (NH4+), which is toxic to aquatic life, into nitrate (NO3+), which is not toxic. A small fraction of phosphorus is used by the microbes for their growth, but most of it remains in solution in the waste water in the form of phosphate ions. In some treatment plants, including Cassington, iron salts are added to precipitate a good fraction of the phosphate and remove it as part of the sludge.
An important fact to note is that there is no attempt to remove nitrate: it remains in the liquid effluent, along with pharmaceuticals, pesticides, microplastics, bacteria, and a heterogenous mix of organic compounds that are refractory to biological digestion. None of these are typically monitored in the effluent of waste water treatment plants in the UK. Thus, even if the plants are operating exactly as intended, they still pour a cocktail of pollutants and bacteria like e-coli into our rivers. Is this the limit of what our present technologies can manage? The answer is, ‘certainly not¨’. Technologies exist for waste-water treatment plants that can produce effluent of near-drinking water quality. The largest Swiss waste-water treatment plant at Zurich is a case in point. Here a large fraction of all the pollutants are removed through successive stages of processing so that the final effluent is ‘clean’ by Swiss standards. And yes, you can even swim in it safely (for details see A Visit to Zurich's Sewage Works). All the technologies involved are well-tested and work at industrial scale. Nothing except political will and cost prevents them being deployed in the UK.

 Zurich's Wastewater Treatment Plant (compare size with Cassington above).
Zurich's Wastewater Treatment Plant (compare size with Cassington above).
But recall that agriculture generates the largest fraction of water pollutants, particularly of nitrate and phosphate. Can anything be done about them? Modern farming practices have substantially reduced the amount of phosphate now used, but the excess phosphate applied to agricultural land over the last 70 years means there remain large 'legacy' reserves of phosphate, which leaches from the soil and enters the ground water and aquifers.
The EU Nitrate Directives sets out steps to reduce the nitrates entering water from farming activities, but the practice can overwhelm the directives. For example, the concentration of the chicken farming industry in Powys, Herefordshire and Shropshire, where at any one time there are 20 million hungry chickens gobbling up phosphate-rich feed and excreting 20 million chicken’s-worth of high-phosphate, high-ammonia excrement, means that even if tools for good governance are in place (which unfortunately is not the case), the eutrophication of the Wye River is inevitable as middens of concentrated pollutants leach from the soil and wash into the river.
On the floodplain, all these issues of pollution come into an even sharper focus, because flooding is the norm and the capacity these meadows provide is buffers and flattens the peaks of the floods. When the arable fields on the floodplain flood, everything that is on the fields washes off: silt, herbicides, fertilizer, pesticides, seed. In the summer floods of 2007, our colleagues at the Floodplain Meadows Partnership at the Open University analysed 10 floodplain meadow sites and found that the flood deposited up to 40 tonnes of sediment per hectare, indicating that a substantial amount of silt can be carried in floodwater.
 Silt from arable fields, Eynsham. Ancient ridge-and-furrow fields visible. Meadows and permanent pastures (left in the photo) do not generate silt, but on the contrary, trap silt - an ancient means of fertilising exploited by the Egyptians.
Silt from arable fields, Eynsham. Ancient ridge-and-furrow fields visible. Meadows and permanent pastures (left in the photo) do not generate silt, but on the contrary, trap silt - an ancient means of fertilising exploited by the Egyptians.
 Silt from arable fields flowing onto meadows downstream of the Toll Bridge road.
Silt from arable fields flowing onto meadows downstream of the Toll Bridge road.
Farmers may choose to play roulette with the weather with their arable crops, but unfortunately the pollution they produce during floods is not confined to their own farms, but spreads far and wide. The run-off from arable fields is brown with silt and since this erosion accompanies every flood, these fields have no soil left, only compacted dirt. Thus, for any crop to grow, the farmer needs to use a great deal of fertilizer. Clearly, the optimal solution is not to plant crops on flood-prone land, but instead farm these fields as permanent pastures or hay meadows (more of which below), both of which ensure the soil is covered with perennial plants to prevent erosion. A compromise solution is to grow cover crops during the seasons most at risk of flooding to reduce the loss of soil. Cover crops like legumes capture nitrogen and create conditions for subsoil fauna, which help to restore the soil, so that less fertilizer is needed for summer crops.
 Long Mead, a fragment of an ancient floodplain wildflower hay meadow.
Long Mead, a fragment of an ancient floodplain wildflower hay meadow.
The NRN is involved in a community-driven initiative whose prime goal is to restore or re-create the intervening meadows between the ancient wildflower meadows in order to create a continuous meadow network along the bend of the Thames around Wytham. Ancient wildflower meadows, like Long Mead (which is recorded in the Domesday Book), are one of the most species-rich patches of land in the UK, but they are also one of the scarcest habitats in the UK – only 4 square miles remain of this plant community, which is about 3% of the original habitat that still existed a century ago. Their plant diversity is a result of their continuous management for agriculture since Saxon times, but hay meadows still play their part in 21st agriculture: Long Mead still does what it has been doing for over 1000 years: providing a crop of hay for winter feed and grazing for sheep and cattle after the hay has been taken. Indeed, in medieval times the floodplain wildflower meadows were the most valuable land in the country, because they did not need additional fertilizing to produce their hay crop – the silt from the floodwater did that – and they provided commons for livestock to graze after the haymaking. We now know that the plant diversity induces a healthy and diverse gut biome in the animals that graze the meadows.
 Great burnet (Sanguisorba officinalis) has roots that are 2-3m deep, so they can store carbon at great depths in the soil. Individial plants may live for 100-150 years. These and the many other perenial meadow plants create a highly structured, porous soil that acts as a sponge to soak up and drain floodwater.
Great burnet (Sanguisorba officinalis) has roots that are 2-3m deep, so they can store carbon at great depths in the soil. Individial plants may live for 100-150 years. These and the many other perenial meadow plants create a highly structured, porous soil that acts as a sponge to soak up and drain floodwater.
The community of perennial plants that has resulted is well-adapted and resilient to flooding, quite unlike the annual crops grown on arable fields, so that regardless of the degree of winter flooding, these wildflower meadows always produce a hay crop and do it without fertilizer, herbicides or pesticides. Conversely, even in the height of a summer drought the meadows remain green, because some species of meadow flowers have meters-long roots that still reach the water table. (In the rush to plant trees to mitigate climate change, we forget that floodplain wildflower meadows are also one of the most effective habitats for sequestering and storing carbon – each hectare of an ancient meadow stores over 200 tonnes of carbon and does so more securely than woodland – and making new meadows can be as satisfying as planting a tree).
In the process of capturing silt, floodplain wildflower meadows also capture pollutants. Due to the perennial plant community, the meadow soil is highly structured and very porous, and it acts like a sponge to absorb floodwater with all its nutrients. Phosphate and nitrate deposited by floodwater are used by the plants for growth and are thus these pollutants are effectively removed from the meadows by means of the annual hay-cut and the grazing of animals. The Floodplain Meadows Partnership estimate that this accounts for the export of 5 kg of elemental phosphorus per hectare every year.
 Harvesting the crop of hay: tedding and baling, Long Mead 2024. Photo K Martin
Harvesting the crop of hay: tedding and baling, Long Mead 2024. Photo K Martin
The hay-cut is not the only way in which the floodplain meadows can remove pollutants, however, for as the water filters through the soil and residual alluvium to the sand and gravel aquifer beneath, microbes in the soil use the nutrients in the water for growth. Microbes that flourish in aerobic layers nitrify the ammonium ions, while another group of microbes in the deeper anerobic layers strip the oxygen atoms from the nitrate (NO3+) and release nitrous oxide (N2O) or nitrogen (N2) to the atmosphere. In this way microbes in the meadow soils ‘denitrify’ the polluted water.
The pH of our meadows is alkaline, and this allows phosphate to be removed from solution by a process called ‘adsorption’, by which reaction the phosphate is removed from solution and incorporated in compounds in the clays in the soil. Phosphate can also be removed by precipitation with iron salts present the ground. These processes limit the availability of free phosphate for growth and so keep the meadow soil relatively nutrient poor, which is essential to maintain the botanic diversity. It is likely that microplastics are also trapped in soil and clay/sand alluvium and are filtered out as the floodwater percolates down to replenish the aquifer, which then compensates for low summer river flows and buffers the rivers against drought.
The floodplain wildflower meadows provide us with so much more than just their unique botanic biodiversity. Without fuss, fanfare, or cost they provide us with long list of ‘eco-system’ services, including the nature-based solution to water pollution discussed here. Clearly, there is no shortage of good reasons to restore floodplain wildflower meadows and we are in the perfect location to do just that - so let’s make more meadows!
Based on a talk given to The Eynsham Society on 20th November 2024.
Kevan AC Martin
